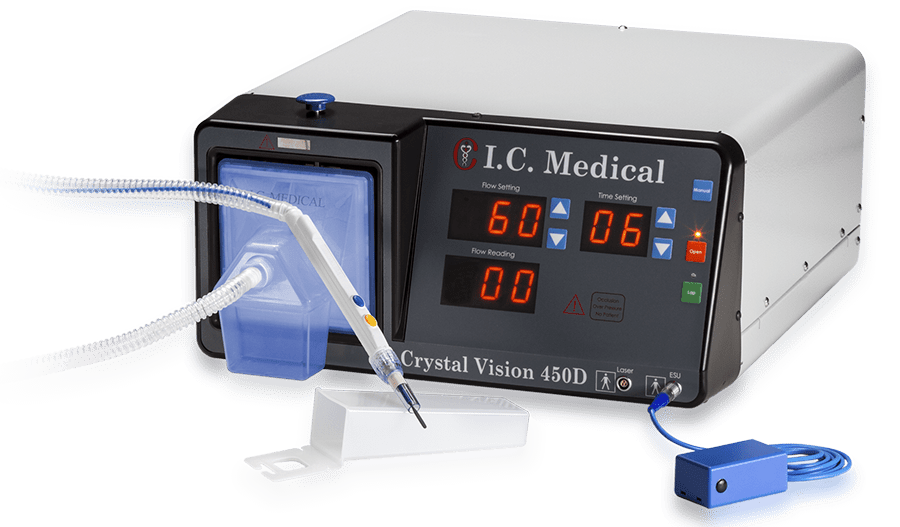
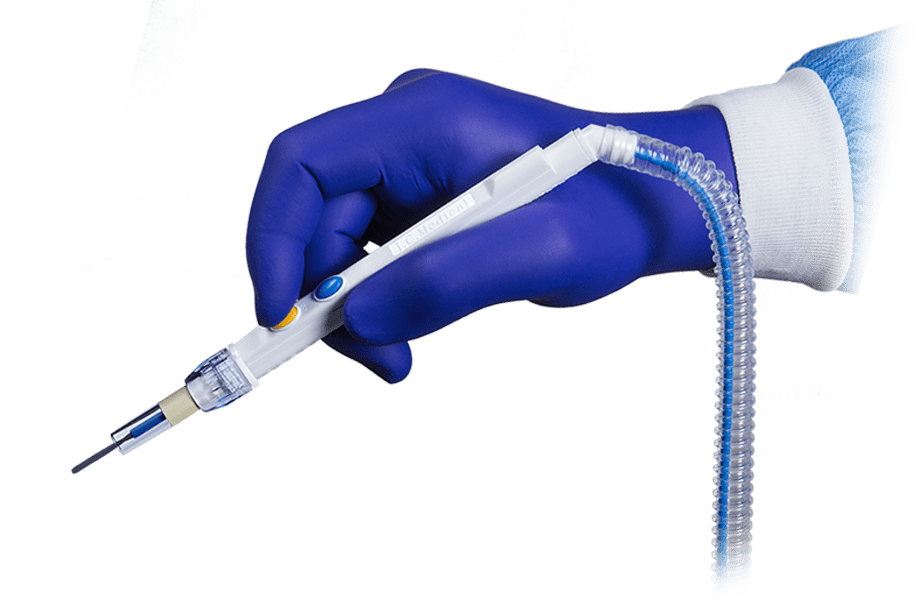
Every I.C. Medical device is produced, assembled, tested and quality assured in-house at the company’s manufacturing facility in Phoenix, AZ.
By maintaining total control over the engineering and production processes, the quality, integrity and reliability of I.C. Medical’s surgical smoke evacuation devices is never compromised.
I.C. Medical’s surgical smoke evacuation systems and accessories undergo rigorous QA and testing procedures to ensure that they are manufactured to the highest standards. All products are made with virgin raw materials sourced from reliable suppliers, and produced and assembled in a pristine manufacturing environment by a well-trained and highly skilled workforce.
Electrosurgical Smoke Hazards
Is electrosurgical smoke safe to inhale? This is the major question, which many surgeons, nurses and branch organizations have been discussing for years. The main cause for worry is the presence of airborne chemicals, viable bacteria, viruses and mutagenic substances. These bacteria and viruses pose a potential risk to the health of the staff in the operating room as revealed in various reports and articles.